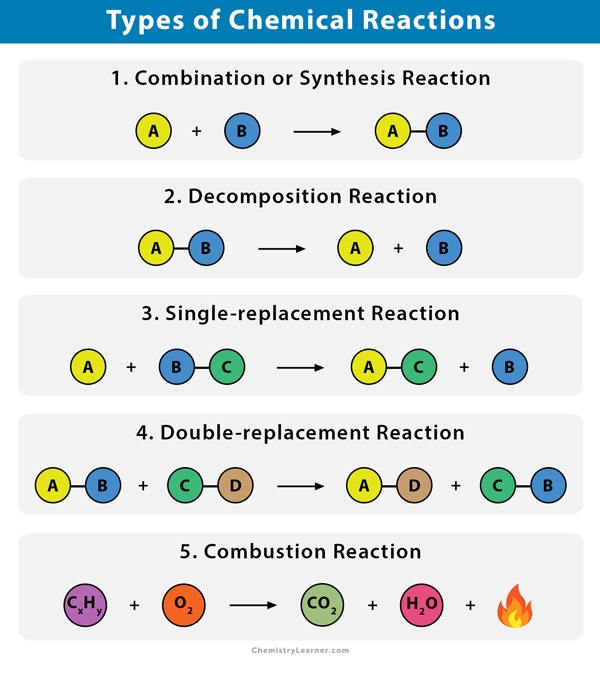
Mg 2HCl MgCl2 H2. A single substance breaks down into more than one substance.
Module 4 Review Chemistry Quizizz
A compound breaks down into separate elements and compounds Rank the following in order of increasing molar mass.

. One element takes place of another in a. Two substances recombine to form two new substances. Two substances recombine to form two new substances c.
Acid base H2O H3O. The reaction for tarnish formation due to contact with hydrogen sulphire 2 A g g H 2 S g A g 2 S s H 2 g and the above tarnish can be removed by using A l metal as 2 A l 3 A g 2 S A l 2 S 3 6 A g The above two reactions appears to be a displacement reaction. Multiple Choice Decomposition reaction Reversible reaction Exchange reaction Synthesis reaction Question.
The correct answer is. One element takes the place of another in a compound. BaCO3 -- BaO CO2.
The the following reaction is. This problem has been solved. Acid base salt water C.
C3H8 g 5O2 g 3CO2 g 4H2O g single displacement reaction. Catalyst drawn lower then original curve. Which of the following best describes a decomposition reaction.
Which of the following word equations describes the chemical reaction that has occurred in this experiment. Three solutions are mixed together to form a single solution. Acid base H OH- B.
Answer this question Which of the following best describes a neutralization reaction. Zn s 2HCl aq 2HCl aq ZnCl2 aq H2 g O single displacement reaction decomposition reaction double displacement reaction O combustion reaction O combination reaction Which of the following correctly describes the Lewis structure molecular geometry. The three general categories of single-replacement reactions are.
Which of the following best describes a decomposition reaction. The chlorine that is released will be in the form of anions. Which of the following best describes the reaction H2CO3 H2O CO2.
All of the above. Which statement describes what happens during a decomposition reaction. Two substances combine to form a single new substance.
Which of the following reaction categories best describes. Which of the following reaction categories best describes. A Hydrogen peroxide water oxygen B Hydrogen peroxide oxygen water C Hydrogen peroxide peroxide hydrogen D Hydrogen peroxide hydrogen oxygen peroxide E.
Proteins encode nucleic acids. Which of the following best. Molecule is broken down Chemical energy is released as bonds are broken.
Which of the following best describes the reaction H2CO3 H2O CO2. Which of the following best describes a balanced reaction. 2 When magnesium is burned in the presence of oxygen it produces magnesium oxide according to the following chemical equation.
This problem has been solved. Two substances combine to form a single new substance b. 62 Manganese dioxide MnO2 sMnO2 s is an insoluble substance that acts as a catalyst for the decomposition reaction.
A reaction that has the same number and type of atoms on each side of the equation. The reaction above is classified as. BaCO3 -- BaO CO2 Decomposition reaction Single replacement reaction Combustion Reaction Synthesis Reaction Double replacement reaction 2 When magnesium is burned in the presence of oxygen Chemistry.
Single-replacement reactions and a double-replacement reactions differ in the fact that in a single-replacement reaction only one element that has a positive charge is oxidized or looses electrons. Acid base metal nonmetal D. Decomposition reaction ABA B synthesis reactions is reverse.
CaCl2 is dissolved in water. 100 1 rating Transcribed image text. See the answer See the answer done loading.
Underlie all catabolic reactions destructive that occur in the body cells. 1 The the following reaction is. H2O2 I2 LiF CH6N.
An experiment shows that hydrogen peroxide decomposes to form water and oxygen gas. Simpler compounds from a complex compound. On the diagram above draw a curve to represent the reaction as it occurs in the presence of MnO2 sMnO2 s.
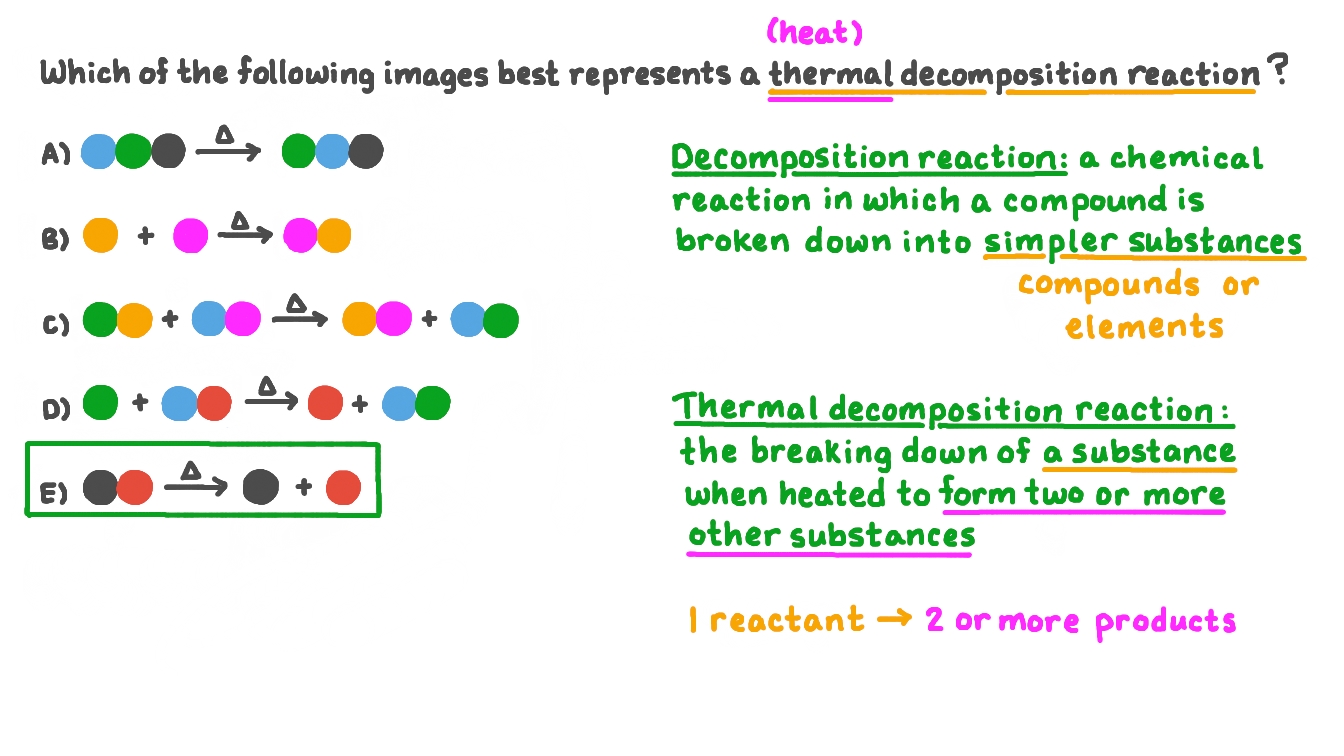
Question Video Identifying An Image That Represents A Thermal Decomposition Reaction Nagwa

Solved Which Of The Following Describes A Decomposition Chegg Com

Decomposition Reactions Youtube Chemical Reactions Chemical Equation Reactions
0 Comments